1-Bromo-3-Methylbutane: From Laboratory Curiosity to Industrial Utility
Historical Development
Chemists in the late nineteenth century started isolating compounds from essential oils and coal tar, often ending up with halogenated byproducts. Researchers noticed that by treating alkanes and alcohols with halogens under certain conditions, they could swap hydrogen for a halogen atom. 1-Bromo-3-methylbutane traces its earliest syntheses to these straightforward substitution reactions. This compound drew growing interest for its role as an intermediate for more structurally complex molecules, especially as industries demanded more advanced synthetic routes in pharmaceuticals, agrochemicals, and flavors. The simplicity of its preparation set a precedent for generations of chemists relying on easily accessible building blocks for organic synthesis.
Product Overview
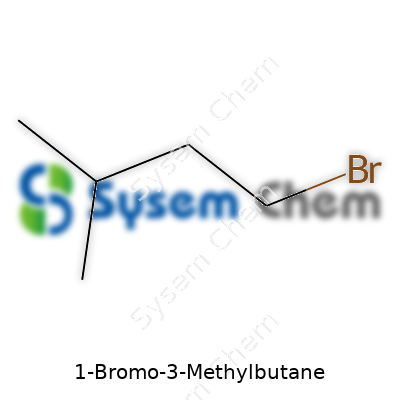
1-Bromo-3-methylbutane belongs to the alkyl bromide family. Its straightforward structure, consisting of a five-carbon backbone punctuated at the third carbon by a methyl group and capped off with a bromine atom, makes it a popular choice for chemists who need a reliable alkylating agent. Its role often centers on adding the isopentyl group to various organic scaffolds. The utility hinges on the bromine's readiness to depart in substitution reactions, which opens doors for forming new carbon-based frameworks. From small-scale exploration in research labs to metric tons in production settings, this chemical has proven itself a reliable workhorse in organic chemistry.
Physical & Chemical Properties
As a clear to pale yellow liquid at room temperature, 1-Bromo-3-methylbutane carries an unmistakable, somewhat sweet odor typical of short-chain alkyl bromides. Its boiling point circles around 118-120°C, with a melting point below the freezing point of water. The molecular weight lands at 151.04 g/mol. Its modest solubility in water can trip up chemists hoping for one-pot aqueous procedures, so most gravitate to organic solvents like diethyl ether, chloroform, and acetone. Density sits at about 1.22 g/cm³, underscoring the presence of bromine. Reactivity stands out — strong nucleophiles will drive clean SN2 substitutions, while base-induced eliminations turn it into more reactive pentenes.
Technical Specifications & Labeling
Industry demands tight tolerances on color, purity, and stability. High-quality 1-bromo-3-methylbutane boasts purity typically upward of 98%, with colorless appearance preferred. Shelf life depends on storage free from moisture and light, as both speed up decomposition and discoloration. Labeling sticks to globally harmonized standards. Containers bear the hazard pictograms warning users of its flammability, moderate acute toxicity, and environmental risks. The packaging, often glass or compatible HDPE, must lock tightly since the liquid evaporates quickly and the vapors pose inhalation hazards.
Preparation Method
Most chemical producers rely on classic nucleophilic substitution of 3-methyl-1-butanol (isoamyl alcohol) with hydrogen bromide. This adaptable method, performed with or without acidic catalysis, often uses concentrated hydrobromic acid or sodium bromide with sulfuric acid. The reaction proceeds under reflux and brisk stirring, maximizing contact between the aqueous and organic layers. After separation and simple purification by distillation, the product reaches purities suitable for research or further industrial transformation. Some outfits push for greener alternatives, minimizing waste and hazardous reagents, but the fundamentals rarely shift far from this proven route.
Chemical Reactions & Modifications
This compound shines as an alkylating agent across a spectrum of reactions. Nucleophiles, from amines to thiols and azides, will eagerly displace the bromine. The resulting products fuel pharmaceutical, fragrance, and agrochemical development. Elimination reactions with strong bases turn it into various pentene isomers, valued for polymer feedstocks and specialty solvents. Its five-carbon skeleton, easily introduced using mild conditions, supports efficient synthesis of branched chain molecules, enhancing structural diversity across synthetic pathways. Functional group exchange, such as swapping the bromine for a hydroxyl using aqueous silver nitrate, allows further manipulation down the line.
Synonyms & Product Names
In the chemical business, many synonyms and trade names cross international borders. Chemists may encounter it as isopentyl bromide or 3-methylbutyl bromide on safety data sheets or container labels. Its systematic IUPAC name, 1-bromo-3-methylbutane, carries across regulatory filings, patent literature, and chemical catalogs. Marketers sometimes lean on its description as a five-carbon bromoalkane to target specialty synthesis markets. An import/export label might display its CAS registry number, 3572-67-6, to eliminate confusion and ensure accurate sourcing.
Safety & Operational Standards
Handling 1-bromo-3-methylbutane safely demands diligence. The liquid easily vaporizes, and inhaling the fumes irritates airways. Prolonged or repeated skin contact causes discomfort or chemical burns, so gloves and goggles serve as first-line defenses. Workplaces handling significant volumes often rely on fume hoods and local exhaust ventilation to control airborne levels. Emergency procedures and spill kits require regular training since brominated organics can contaminate water and soil. Waste management includes collecting used chemicals and tainted gear in designated hazardous waste streams, tracked by environmental authorities. Safety data documentation remains thorough, alerting staff to fire risks and reactivity with strong bases or oxidizers.
Application Area
This chemical sits in the toolkits of those in pharmaceuticals, specialty polymers, agrochemicals, and flavor chemistry. Its ability to install the isopentyl group gives it value in building blocks for antihistamines, anticonvulsants, and certain antiretroviral agents. The flavor and fragrance industry taps it, or its derivatives, for fruity and banana-like notes that enhance consumer products. As a precursor to branched alkanes or alkenes, it finds a place in manufacturing functionalized polymers, particularly where branched side-chains boost flexibility or change compatibility. Chemical developers favor it for introducing complexity into otherwise linear carbon scaffolds.
Research & Development
Research chemists keep returning to 1-bromo-3-methylbutane for new coupling reactions, asymmetric alkylations, and C–C bond formations. Advances in transition metal catalysis and greener synthetic techniques often take this chemical as a reliable substrate, ensuring reproducibility and scalability. Newer work explores activating otherwise inert C–H bonds adjacent to the isopentyl group, aiming for shorter synthetic sequences or late-stage diversification in drug candidates. Researchers also look for bio-based or recyclable reagents to streamline the synthesis, cutting down the need for hydrobromic acid or harsh conditions.
Toxicity Research
Early studies documented acute toxicity from dermal exposure, inhalation, or ingestion, leading to central nervous system depression or liver stress. Researchers in toxicology continue to map out metabolic breakdown and long-term exposure risks at both laboratory and environmental levels. Animal testing reveals dose-linked effects such as drowsiness or mild respiratory distress, pushing manufacturers to site-specific exhaust and monitoring for workplace exposure. Environmental studies explore its fate in water and soil, tracking how quickly microbes or sunlight degrade the compound. Authorities set occupational exposure limits to ensure worker safety, and consumer product rules keep it away from direct contact goods.
Future Prospects
Growth in organic electronics, medicinal chemistry, and greener synthesis creates room for innovation. As chemical engineers aim to swap hazardous agents for safer or more sustainable options, the basic chemistry underpinning alkyl bromide synthesis faces ongoing evaluation. Researchers experiment with bio-derived isoamyl alcohol from fermentation, cutting reliance on petrochemicals and lessening environmental impact. Computational modeling and high-throughput screening continue to unlock new types of alkylation and substitution chemistry using 1-bromo-3-methylbutane. Regulatory attention keeps tightening around toxic byproducts and emissions, pressing producers to adapt manufacturing and purification. Despite these changes, chemists still bet on this simple, versatile compound for reliable transformations across diverse frontiers.
The Building Block for Bigger Ideas
1-Bromo-3-methylbutane sounds like something pulled straight from a chemistry exam, but in labs and industry, it’s a core player. This compound acts as a reliable alkylating agent. Chemists count on it to add a carbon chain right where it’s needed in a larger molecule. That carbon-bromine bond easily breaks and allows the molecule to link up with others. This comes in handy for building pharmaceuticals—especially drugs based on complex organic structures. Many of those start with molecules just like this one, which help create the framework before tailoring them for things like pain relief or infection-fighting power.
Making Flavors, Fragrances, and a Lot More
It’s not just big pharma relying on this chemical. The fragrance and flavor industries reach for 1-bromo-3-methylbutane as a starter molecule. The structure lets chemists create subtle variations in taste and scent. Building new flavors and fragrances means tweaking molecules until they produce just the right note—whether it’s adding depth to a vanilla substitute or helping a floral perfume linger a little longer. If you’ve ever found a synthetic food flavor almost uncannily close to the real thing, odds are someone got there with building blocks derived from chemicals like this.
Fuel Additives and Beyond
1-Bromo-3-methylbutane also finds its way into fuel chemistry. Some specialty fuels use it or its derivatives as intermediates in octane boosters or antiknock agents. These chemicals keep engines running smoothly, especially when regulations or technology demand cleaner-burning and more efficient gasoline. People don’t see this work, but it improves how cars run and reduces mechanical wear over time.
Pushing the Science Forward
I remember plenty of time spent in college labs with compounds like this. Before seeing it in action, a lot of us wrote off these small, odd-sounding chemicals. The moment you add it to a reaction and watch a foggy white solid form or a pungent liquid separate out, you realize how much backbone they give to modern research. Researchers aren’t just creating medicines or materials—they’re working with molecular pieces that require absolute confidence. If an intermediate like 1-bromo-3-methylbutane doesn’t meet expectations, the whole chain falls apart.
Safety and Environmental Responsibility
Handling 1-bromo-3-methylbutane takes some respect for safety. Its volatility means it isn’t forgiving if spilled or mishandled. Breathing its vapors, even for a short time, leads to health risks. Labs and factories need well-ventilated environments and personal protective equipment. Regulations on these chemicals keep them in check, but there’s always room for better storage and disposal options. Sometimes innovation means making these steps safer for the workers and for the environment around manufacturing sites. Seeing how spill management has changed in academic labs over the years, training and awareness have made a clear difference, but ongoing diligence matters.
Thinking About the Future
People developing greener chemistry take a close look at substances like 1-bromo-3-methylbutane. Safer alternatives for some of its jobs remain a work in progress, but progress keeps coming. Cleaner, bio-based sources might someday provide the same chemical backbone with less impact. Until that future arrives, careful regulation, transparent safety data, and a culture of respect in handling remain key.
1-Bromo-3-methylbutane sits in the space where science meets daily life. Its footprint stretches from perfumers’ labs to refineries and pharmaceutical research. The more we respect both its power and its risks, the more we get out of this humble molecule—and the more safely we’ll move ahead.
Stepping Into Organic Chemistry: A Commonly Used Alkyl Bromide
A bottle labeled 1-Bromo-3-Methylbutane brings me back to university days, breathing in the sharp scent of solvents and hearing the buzz of overhead lights in the lab. This compound, a clear example of a brominated alkane, puts simple molecular building blocks on display without much pretense. The formula sums it up: C5H11Br.
Breaking Down the Molecular Formula
Thinking about the name tells a lot about the structure. Five carbons, eleven hydrogens, and a bromine atom make up the skeleton. It starts with “butane” as the root, and the “3-Methyl” describes a one-carbon branch on the third carbon. The “1-Bromo” says the bromine attaches to the first carbon. Many chemists mark brominated alkanes by their ability to serve as handy intermediates — that is, they help make bigger, more complicated molecules.
The Structure, Drawn Simply
Chemists see it like this: start with a straight five-carbon chain. Number the carbons one through five. Put a bromine on carbon one. Shift to carbon three, where a methyl group (CH3) branches off. Lay it out flat:
Br–CH2–CH2–CH(CH3)–CH3
Visual learners might imagine a zigzag line with a “Br” stuck at one end, and a “CH3” helicoptering off somewhere in the middle.
Why 1-Bromo-3-Methylbutane Finds Its Way Into Labs
In teaching labs, this compound shows how substitution reactions work. Its straightforward structure lets beginners map out which atoms belong where, and predict what happens when bases or nucleophiles step in. Synthetic chemists turn to it for its reliability. For example, introducing the bromine atom makes the molecule more reactive. Put simply, the bromine acts as a leaving group – something that pops off, making way for new parts to join the molecule. This is crucial for making pharmaceuticals, agrochemicals, or even more complicated organic molecules.
Knowing the structure reveals more than a sum of atoms. The arrangement creates physical properties – a boiling point around 90 to 91°C, a dense and somewhat oily feel, and a faintly sweet aroma once you get past the expected brominated sharpness. Handling safety sits up front; bromine atoms mean you wear gloves and work under a fume hood, not tossing caution aside like a TV scientist.
Challenges and Looking for Better Solutions in the Lab
Reactions with compounds like 1-Bromo-3-Methylbutane push for careful attention to waste and safety. Brominated compounds do not break down gently in nature, so disposal takes planning. Labs committed to green chemistry now hunt for alternatives, sometimes shifting to less persistent halides or fully different leaving groups, aiming to cut down hazardous waste. Inventive minds have moved toward catalytic strategies or using recyclable reagents. Keeping the beautiful simplicity of the molecule, while dropping the environmental baggage, marks real progress.
Learning From Classic Alkyl Bromides
Chemistry professors like to say that once you see the logic in a small molecule, the sprawling worlds of drug design and material science look a little less daunting. 1-Bromo-3-Methylbutane builds that foundation. Recognizing every part of its structure, respecting its risks, and striving for cleaner chemistry mirror how science keeps moving, problem by problem, molecule by molecule.
The Substance on the Table
People in chemical labs, college classrooms, and some industries deal with 1-Bromo-3-Methylbutane when synthesizing new compounds or carrying out research. While its formula might look straightforward, this liquid brings more risk than beginner chemists often realize. Whenever someone mentions brominated organics, I remember my first lab internship, when a small splash from a similar compound led to a headache and a real appreciation for respect in the workplace.
Skin and Inhalation Dangers
Direct skin contact brings irritation, redness, or even blisters after long exposure. Every time you use this chemical, don’t even consider skipping gloves. Nitrile gloves stop most of the trouble, and a lab coat adds important protection. No lab should allow open-toe shoes or loose sleeves. Splashes have a way of finding lazy habits.
At room temperature, fumes can form quickly. Without proper ventilation, even a tiny spill leaves a pungent odor and risk for headaches, nausea, and respiratory problems. This is not just a lab myth— a friend spent three hours with dizziness after opening a bottle outside the fume hood. The fume hood is not just a box; it saves your lungs and, if used consistently, prevents contamination in shared spaces. A common fan doesn’t cut it.
Goggles, Not Just Glasses
Regular glasses won't protect your eyes from splashes, so safety goggles glass up every session. One time, I saw a small drop bounce off a bench and catch a lab partner in the eyebrow. Even a drop in the eye means urgent medical help, so don’t take shortcuts on this rule.
Good Habits: Storage and Labeling
Storing 1-Bromo-3-Methylbutane means choosing a cool, dry place, out of sunlight and away from heat or sparks. It’s flammable, and a forgotten bottle next to a heating element almost caused a fire at my old lab. Secure lids, tight labeling, and dedicated chemical cabinets keep confusion out of the picture. Never reuse a bottle for water or anything else— mistakes happen fast.
Spills and Cleanups
Small spills sound simple, but improper handling can linger for weeks. Absorbent material, a little patience, and safe disposal turn a bad moment into a safe one. Ventilate the area and never sweep spills toward sinks. Waste from this chemical won’t just flush away safely. Label spill kits and let everyone in the lab know where they live.
Disposal Rules
Mixing chemicals down the drain never solves anything. Local rules, hazardous waste containers, and specialized pickup keep people and groundwater safe. One visit to a hazardous waste facility opens your eyes to how much overlooked dumping damages the environment.
Respect Over Routine
Care with 1-Bromo-3-Methylbutane boils down to respect— for yourself, for coworkers, and for the safety of everyone sharing the space. Someday a mistake might tempt someone to shrug and carry on, but taking an extra minute means less worry for everyone. Quality habits keep small accidents from spiraling into big ones, and the right gear feels much lighter than regret or injury.
What is 1-Bromo-3-Methylbutane Used For?
If you've spent any time in an organic lab, you know 1-Bromo-3-methylbutane. It plays a role in making other chemicals, especially when building blocks with carbon and halogen links are needed. It often turns up in university labs and some industrial shops that prepare molecules for medicines or flavors. Not every home pantry keeps a bottle, but chemists see it as a trusty stepping stone. But there's a catch—it brings risk along with usefulness.
Conditions to Store This Chemical
This compound, clear as water and sharp in smell, doesn’t forgive carelessness. If kept in the wrong place or handled poorly, it can spark headaches, dizziness, or, in some cases, cause organ damage over time. Leaks or spills can harm not just the lab workers, but anyone sharing the air. Science has confirmed that bromine compounds, in general, don’t get along well with moisture or high heat. They like to sneak out of unsealed bottles, slowly filling a room with dangerous fumes. So, how do you keep it safe? Old hands in the lab rely on a set of rules passed down year after year.
Storing 1-Bromo-3-methylbutane With No Regrets
First, use only containers made for chemicals. Glass with a strong, tight-fitting cap usually beats out any plastic. Avoid anything that might break down or react with halogenated compounds. Always label each bottle, right down to hazard warnings and the date you started using it. This routine may seem like overkill, but those details save lives. I’ve lost count of how many times a messy shelf almost became a trip to the ER.
Keep 1-Bromo-3-methylbutane away from any flame or heat source. Even simple things like a coffee-maker or a power strip can provide enough heat to start a slow breakdown, raising pressure in the bottle. Over time, that leads to leaks or, in the worst-case, a bottle that bursts. Store it in a cool, dry, and well-ventilated area— a chemical flammables cabinet with a vent takes the prize. Never let it share a shelf with strong oxidizers, acids, or bases. Few things explode as quickly as brominated organics that meet peroxides or concentrated acid. A single splash can wreck the setup and injure everyone nearby.
Shelf Life and Inspections
Chemical safety doesn’t end at storage. Regularly check bottles for cloudiness, color changes, or bulging caps. These signs suggest a reaction has started inside or contamination happened somewhere along the line. Toss anything that looks suspicious following hazardous waste rules. Nobody wants to discover an old bottle hissing under pressure after five years gathering dust.
Training and Awareness Help More Than Technology
Hazard communication—clear labels, up-to-date data sheets, daily reminders—matters more than just about any fancy equipment. In my own lab days, a simple rundown of the rules made all the difference for the new students. Make safety habits routine and keep the emergency spill kit close at hand. Most accidents happen due to old habits or just forgetting how easy it is for a mistake to slip in, especially when days run long and busy.
Responsible Action
The safest labs I’ve seen invest as much in proper storage as they do in training. Store 1-Bromo-3-methylbutane with the same care you’d use for fire or sharp blades. Easy access to accident logs, data sheets, and disposal procedures creates a living culture of safety, not just a list of rules taped to a wall.
Boiling Point: A Key Detail for Handling
Boiling point speaks volumes about a chemical’s uses and storage needs. 1-Bromo-3-methylbutane boils at about 91 to 92 degrees Celsius. This number may not seem dramatic, but it matters. In the world of chemicals, substances that boil below water get extra attention. A low boiling point can make a liquid more volatile and easier to lose through evaporation. In a busy lab, someone who’s distilling 1-Bromo-3-methylbutane can separate it cleanly at this temperature, well before many impurities come into play.
What does this mean outside of the lab? Safety stands front and center. Breathing in vapors can irritate the respiratory tract, so working in a hood acts as common sense. For those storing it, strong seals and cool, dark storage prevent loss and spill risks. Situations where solvents break free, even just a few degrees above room temperature, demand respect.
Density: Heavier Than You Might Guess
1-Bromo-3-methylbutane weighs in at roughly 1.19 grams per cubic centimeter at 20°C. Go ahead—drop a little in water, and it sinks fast. This density springs from its bromine atom. Bromine atoms tip the scale. Compared to something like hexane (which floats), 1-Bromo-3-methylbutane settles down at the bottom in a two-phase mixture.
Chemists rely on this property when they wash or extract substances. Layer separation gets easier to predict, mistakes fall off. If you’ve ever tugged at sticky molecular layers in a separating funnel, you know the value of a good density difference. Good information saves time and spares frustration.
Implications in Laboratories and Industry
Running a reaction that uses 1-Bromo-3-methylbutane, the boiling point signals right away how gentle or aggressive heating should go. Overheating means loss of material and potential safety incidents. For industries that use this material as a chemical building block, understanding boiling point and density allows engineers to design safe equipment, gauge the need for ventilation, and select reactor materials that resist corrosion.
Anyone working with halogenated compounds quickly learns to respect the increased risks. Brominated molecules often carry more environmental baggage than their hydrocarbon cousins. They linger longer. Waste must get managed with real care—casual dumping would lead to persistent pollution. Good practice calls for dedicated collection bins and responsible disposal, not just for legal reasons but out of respect for health and environmental legacy.
Safety and Environmental Considerations
Handling volatile organobromines isn’t just a job for scientists in white coats. Factory workers, students, and mechanics—everyone deserves to know about the real risks behind the numbers. Proper gloves, tight-sealing flasks, fresh goggles, and patience during transfers are musts. Simple lessons and good signage help cut the temptation to take shortcuts.
Facilities that take safety seriously organize regular auditing of chemical storage and emphasize prompt cleanup of spills, especially for heavier-than-water solvents like 1-Bromo-3-methylbutane. Regulatory bodies encourage periodic environmental sampling near plants processing bromoalkanes. This helps to catch leaks before they grow into costly problems.
Making the Numbers Work for Us
Far from being cold statistics, the boiling point and density of 1-Bromo-3-methylbutane shape every approach you take—from reaction planning to waste management. Learning about the physical properties of key reagents shouldn’t stop at chemistry courses. Real-world choices and habits get better with clear-headed attention to numbers like these.